Mechanical stimulation and electrophysiological monitoring at sub-cellular resolution
Synchronized combination of atomic force microscopy, high-density microelectrode array, and fluorescence microscopy developed. System can mechanically characterize and stimulate individual neurons at picoNewton force sensitivity and nanometer precision while monitoring electrophysiological activity at sub-cellular spatial and millisecond temporal resolution.
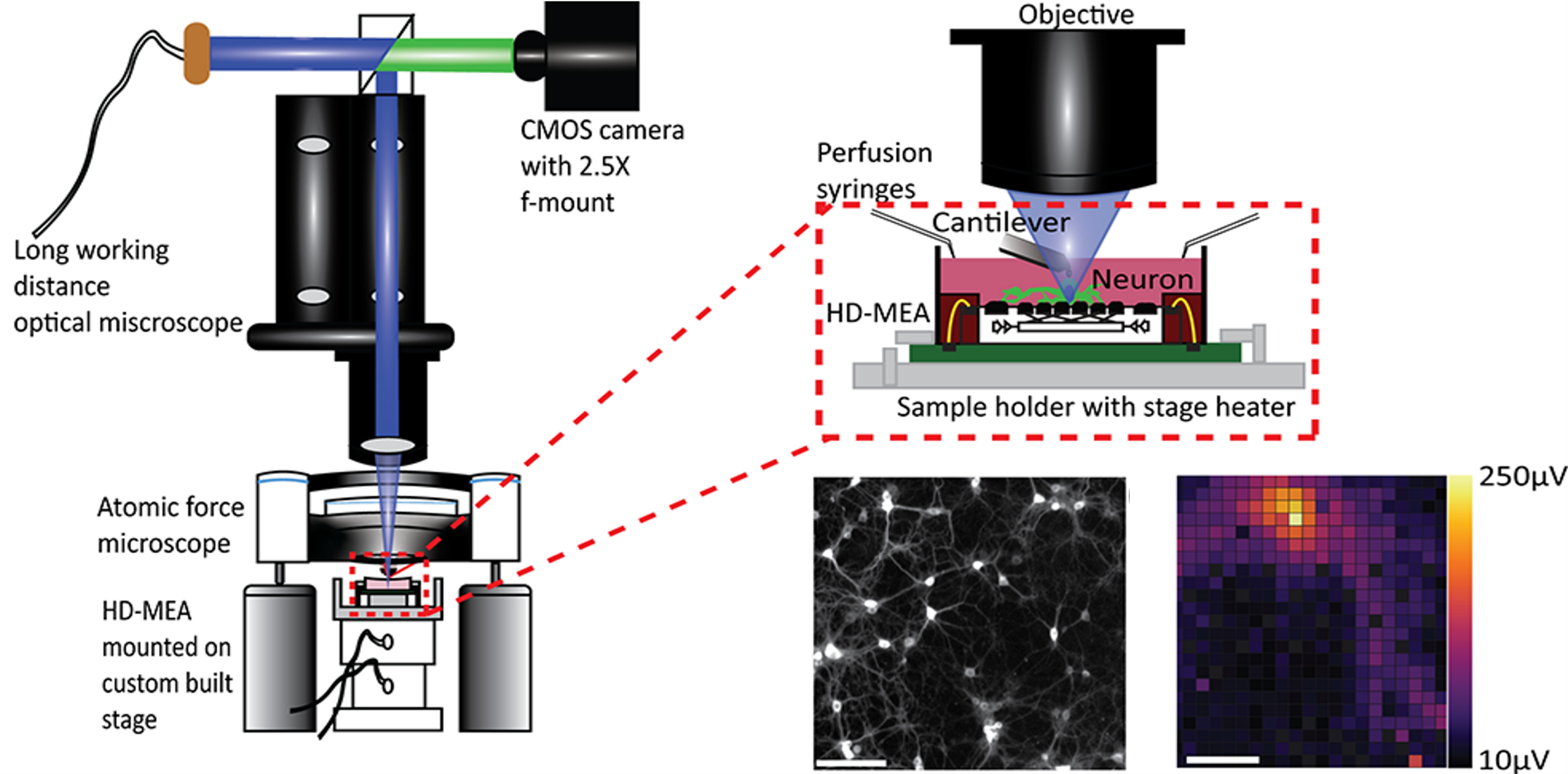

Growing consensus that the brain is a mechanosensitive organ drives the need for tools that can simultaneously mechanically stimulate and record the electrophysiological response of neurons in neuronal networks. We developed a synchronized combination of atomic force microscopy, high-density microelectrode arrays, and fluorescence microscopy to address this challenge (K. Kasuba, A. Buccino, J. Bartram, et al., "Mechanical stimulation and electrophysiological monitoring at subcellular resolution reveals differential mechanosensation of neurons within networks", Nature Nanotechnology 2024 (DOI: 10.1038/s41565-024-01609-1)). We used the unique assay to select individual neurons and to mechanically characterize and stimulate them at picoNewton force sensitivity and nanometer precision while monitoring their electrophysiological activity at sub-cellular spatial and millisecond temporal resolution.
No correlation was found between mechanical stiffness and electrophysiological activity of neuronal compartments. However, local application of transient mechanical stimuli to the neuronal soma was found to evoke action potentials. Neurons showed higher responsivity, including bursts of action potentials, to slower transient mechanical stimuli. The mechanically evoked action potentials depended on the anchoring of the neuronal membrane to the actin cytoskeleton. Furthermore, we observed that spontaneously active neurons showed exceptional functional resilience to static mechanical compression of their soma, whereas a transient and repetitive application of the same compression modulated their firing rate. Seemingly, neuronal networks can differentiate and respond to specific characteristics of mechanical stimulation.
external pageLink to journal paper
