Directed evolution
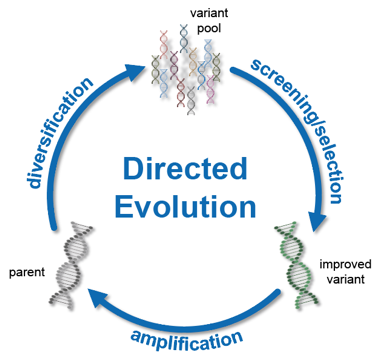
Directed evolution is a bioengineering approach for modifying the properties of biomolecules. Inspired by Darwinism, directed evolution employs an accelerated cycle of genetic diversification (mutation) and subsequent isolation improved mutants (selection). This iterative process allows for the retrieval of genetic variants with improved performance for desired tasks and operational conditions, both of which can be simply dictated by the experimenter. Critical factors that determine the outcome of directed evolution efforts are the availability of high-quality mutant libraries and robust methods to assess large numbers of individual variants (i.e. screening), the latter of which represents an important limiting factor of directed evolution.
In the Bioprocess Laboratory, we address these challenges of directed evolution by developing novel in vivo and in vitro methods for diversity generation and high-throughput screening or selection. Moreover, we apply directed evolution to endow biological systems with new-to-nature functions ranging from individual components(peptides, proteins, enzymes etc.) to more complex systems (pathways, organisms).
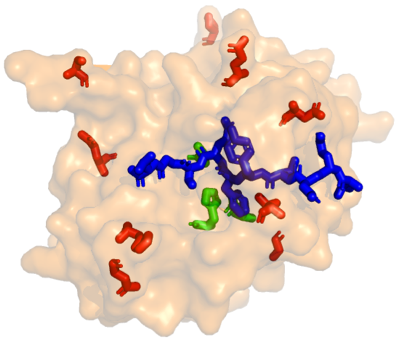
We have applied directed evolution methods to various protein scaffolds (enzymes, binding proteins, fluorescent proteins) to engineer them for various applications. For example, we have employed a semi-rational (iterative saturation) mutagenesis strategy and an HPLC-based screening assay to increase the activity and thermo-stability of epimerases for rare sugar production.
An ongoing engineering project in the Bioprocess Laboratory deals with the engineering of proteases for targeted removal of tagged proteins from cell free extracts.
References
A. Bosshart, S. Panke and M. Bechtold: Systematic optimization of interface interactions increases the thermostability of a multimeric enzyme, Angewandte Chemie International Edition, 2013, external pageDOIcall_made.
S. Oesterle, T.M. Roberts, L.A. Widmer, H. Mustafa, S. Panke and S. Billerbeck: Sequence-based prediction of permissive stretches for internal protein tagging and knockdown, BMC Biology, 2017, external pageDOIcall_made.
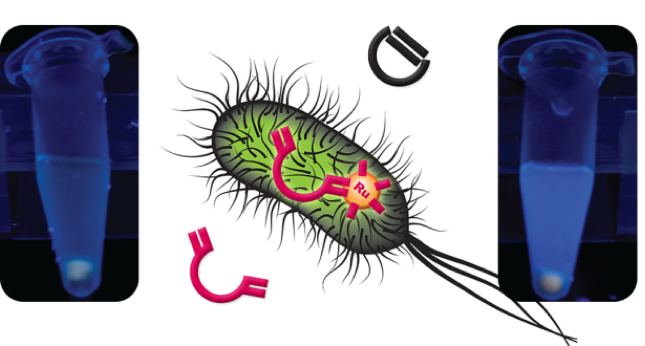
We also employ the tools of directed evolution for the development and optimization of artificial metalloenzymes (ArMs) based on the streptavidin-biotin technology. These hybrid biocatalysts contain artificial transition metal cofactors embedded in a protein scaffold enabling them to carry out functions not found in nature. To this end, we have recently evolved a ruthenium-containing ArM for olefin metathesis in the periplasm of E. coli. This was achieved by engineering residues around the catalytic center of the ArM using iterative saturation mutagenesis in combination with a microtiter plate fluorometric whole-cell assay.
References
M. Jeschek, S. Panke and T.R. Ward: Artificial metalloenzymes on the verge of new-to-nature metabolism, Trends in Biotechnology, 2017, external pageDOIcall_made.
M. Jeschek, R. Reuter, T. Heinisch, C. Trindler, J. Klehr, S. Panke and T.R. Ward: Directed evolution of artificial metalloenzymes for in vivo metathesis, Nature, 2016, external pageDOIcall_made.
M. Jeschek, S. Panke and T.R. Ward: Periplasmic screening for artificial metalloenzymes, Methods in Enzymology, 2016, external pageDOIcall_made.
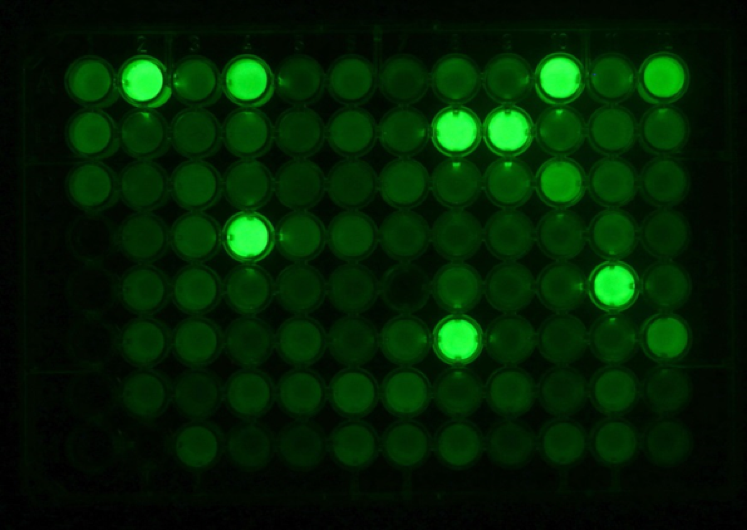
We have also been successful in engineering proteins that do not function as enzymes, e.g. fluorescent proteins. By employing several rounds of random mutagenesis and gene shuffling we were able to identify a bright and pH-stable green fluorescent protein which enables monitoring of cellular processes that occur at low pH.
We are currently working on the development of novel antimicrobial peptides via recombineering and the refactoring of a small molecule-binding transcriptional regulator proteins, critical constituents of biosensor circuits used in high-throughput enzyme/metabolic screening protocols.
References
T.M. Roberts, F. Rudolf, A. Meyer, R. Pellaux, E. Whitehead, S. Panke and M. Held: Identification and Characterisation of a pH-stable GFP, Scientific Reports, 2016, external pageDOIcall_made.
