Microphysiological drug-testing platform for leukemia
Microphysiological drug-testing platform developed that enables co-culturing and controlled interaction of patient-derived leukemia cells, human bone marrow mesenchymal stromal cells, and human liver microtissues within the same microfluidic system.
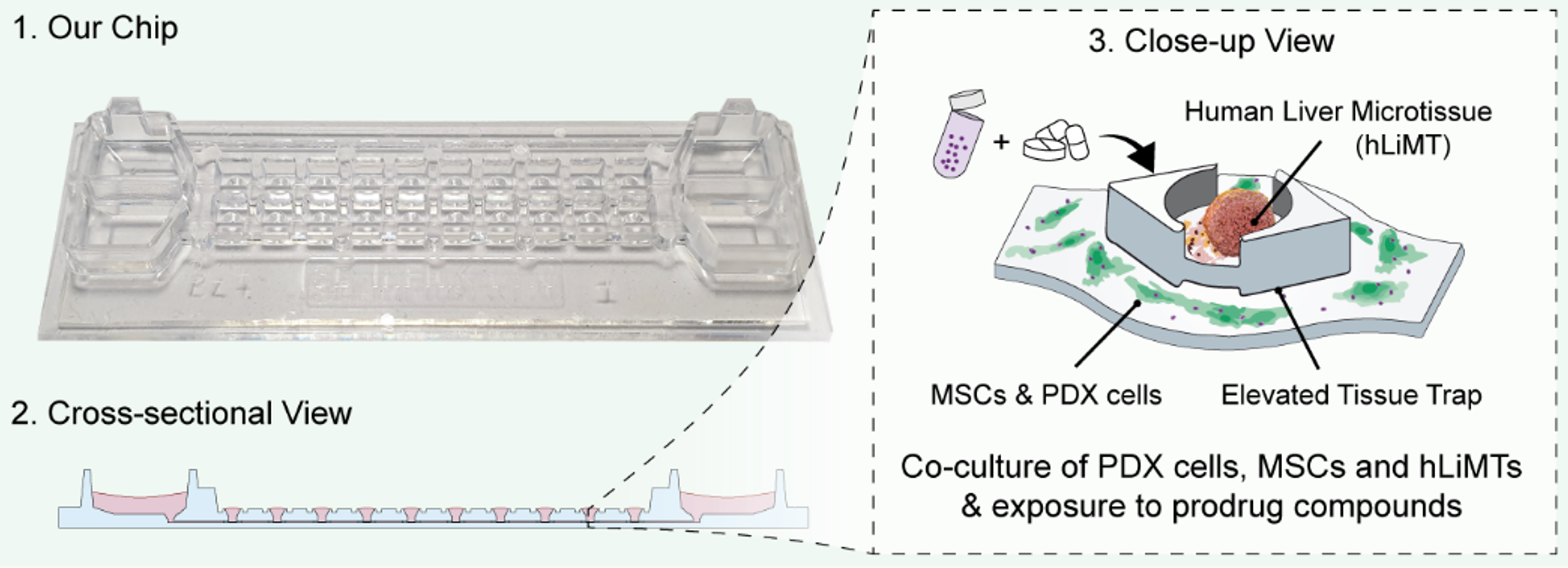

Despite increasing survival rates of pediatric leukemia patients over the past decades, the outcome of some leukemia subtypes has remained dismal. Drug sensitivity and resistance testing on patient-derived leukemia samples provide important information to tailor treatments for high-risk patients. However, currently used well-based drug screening platforms are severely limited in predicting the effects of prodrugs, a class of therapeutics that require metabolic activation to become effective. To address this limitation, a microphysiological drug-testing platform was developed that enables co-culturing of patient-derived leukemia cells, human bone marrow mesenchymal stromal cells, and human liver microtissues within the same microfluidic platform that - at the same time - regulates the physical interaction between the diverse cell types (F. Gökce et al., "Microphysiological drug-testing platform for identifying responses to prodrug treatment in primary leukemia", Advanced Healthcare Materials 2023, Article 2202506). The model recapitulates hepatic prodrug activation of ifosfamide, which cannot be assessed in traditional well-based assays. By testing the susceptibility of primary patient-derived leukemia samples to the prodrug ifosfamide, sample-specific sensitivities to ifosfamide were identified . The developed microfluidic platform enables the recapitulation of physiologically relevant conditions and the testing of prodrugs that yield short-lived and unstable metabolites.
